The Most Electronegative Atom
The elements of the periodic table sorted by electronegativity
click on any element's name for further chemical properties, environmental data or health effects.
This list contains the 118 elements of chemistry.
| The chemical elements of the periodic chart sorted by: | Electro- negativity | Name chemical element | Symbol | Atomic number |
| - Name alphabetically | 0,7 | Actinium | Ac | 89 |
| - Atomic number | 0,79 | Lanthanum | La | 57 |
| - Symbol | 0,82 | Potassium | K | 19 |
| - Atomic Mass | 0,82 | Strontium | Sr | 38 |
| - Electronegativity | 0,89 | Cerium | Ce | 58 |
| - Density | 0,89 | Thorium | Th | 90 |
| - Melting point | 0,93 | Sodium | Na | 11 |
| - Boiling point | 0,95 | Yttrium | Y | 39 |
| - Vanderwaals radius | 0,98 | Lithium | Li | 3 |
| - Year of discovery | 1 | Potassium | K | 19 |
| - Inventor surname | 1,1 | Praseodymium | Pr | 59 |
| - Elements in earthcrust | 1,1 | Protactinium | Pa | 91 |
| - Elements in human body | 1,12 | Neodymium | Nd | 60 |
| - Covalenz radius | 1,13 | Promethium | Pm | 61 |
| - Ionization energy | 1,14 | Samarium | Sm | 62 |
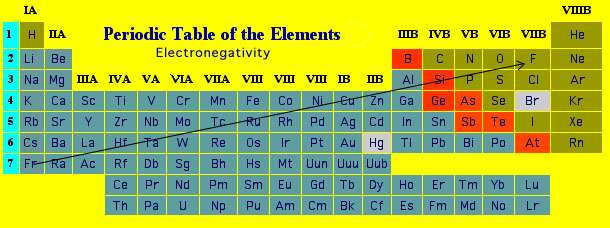
For chemistry students and teachers: The tabular chart on the right is arranged by electronegativity. The first chemical element is Actinium and the last element is Fluorine. The unity used for the electronegativity is Pauling. Please note that the elements do not show their natural relation towards each other as in the Periodic system. There you can find the metals, semi-conductor(s), non-metal(s), inert noble gas(ses), Halogens, Lanthanoides, Actinoids (rare earth elements) and transition metals. | ||||
| 1,17 | Gadolinium | Gd | 64 | |
| 1,2 | Dysprosium | Dy | 66 | |
| 1,22 | Zirconium | Zr | 40 | |
| 1,22 | Erbium | Er | 68 | |
| 1,23 | Thulium | Tm | 69 | |
| 1,24 | Ytterbium | Yb | 70 | |
| 1,25 | Lutetium | Lu | 71 | |
| 1,27 | Tantalum | Ta | 73 | |
| 1,28 | Curium | Cm | 96 | |
| 1,3 | Tungsten | W | 74 | |
| 1,3 | Uranium | U | 92 | |
| 1,3 | Berkelium | Bk | 97 | |
| 1,3 | Californium | Cf | 98 | |
| 1,3 | Einsteinium | Es | 99 | |
| 1,3 | Fermium | Fm | 100 | |
| 1,3 | Mendelevium | Md | 101 | |
| 1,3 | Nobelium | No | 102 | |
| 1,3 | Lawrencium | Lr | 103 | |
| 1,3 | Rutherfordium | Rf | 104 | |
| 1,3 | Dubnium | Db | 105 | |
| 1,31 | Magnesium | Mg | 12 | |
| 1,33 | Niobium | Nb | 41 | |
| 1,36 | Calcium | Ca | 20 | |
| 1,36 | Americium | Am | 95 | |
| 1,38 | Plutonium | Pu | 94 | |
| 1,5 | Rhenium | Re | 75 | |
| 1,5 | Neptunium | Np | 93 | |
| 1,54 | Scandium | Sc | 21 | |
| 1,55 | Chromium | Cr | 24 | |
| 1,57 | Beryllium | Be | 4 | |
| 1,6 | Molybdenum | Mo | 42 | |
| 1,61 | Aluminum | Al | 13 | |
| 1,62 | Bismuth | Bi | 83 | |
| 1,63 | Titanium | Ti | 22 | |
| 1,65 | Copper | Cu | 29 | |
| 1,66 | Vanadium | V | 23 | |
| 1,69 | Indium | In | 49 | |
| 1,78 | Tin | Sn | 50 | |
| 1,81 | Zinc | Zn | 30 | |
| 1,83 | Manganese | Mn | 25 | |
| 1,88 | Iron | Fe | 26 | |
| 1,9 | Silicon | Si | 14 | |
| 1,9 | Nickel | Ni | 28 | |
| 1,9 | Ruthenium | Ru | 44 | |
| 1,9 | Iridium | Ir | 77 | |
| 1,91 | Cobalt | Co | 27 | |
| 1,93 | Cadmium | Cd | 48 | |
| 1,96 | Antimony | Sb | 51 | |
| 2 | Lead | Pb | 82 | |
| 2 | Radon | Rn | 86 | |
| 2,01 | Gallium | Ga | 31 | |
| 2,02 | Astatine | At | 85 | |
| 2,04 | Boron | B | 5 | |
| 2,05 | Iodine | I | 53 | |
| 2,1 | Xenon | Xe | 54 | |
| 2,16 | Technetium | Tc | 43 | |
| 2,18 | Arsenic | As | 33 | |
| 2,19 | Phosphorus | P | 15 | |
| 2,2 | Hydrogen | H | 1 | |
| 2,2 | Rhodium | Rh | 45 | |
| 2,2 | Silver | Ag | 47 | |
| 2,2 | Platinum | Pt | 78 | |
| 2,2 | Gold | Au | 79 | |
| 2,2 | Francium | Fr | 87 | |
| 2,28 | Palladium | Pd | 46 | |
| 2,28 | Mercury | Hg | 80 | |
| 2,33 | Polonium | Po | 84 | |
| 2,36 | Osmium | Os | 76 | |
| 2,54 | Thallium | Tl | 81 | |
| 2,55 | Carbon | C | 6 | |
| 2,55 | Selenium | Se | 34 | |
| 2,58 | Sulfur | S | 16 | |
| 2,6 | Barium | Ba | 56 | |
| 2,66 | Cesium | Cs | 55 | |
| 2,96 | Krypton | Kr | 36 | |
| 3,04 | Nitrogen | N | 7 | |
| 3,16 | Chlorine | Cl | 17 | |
| 3,44 | Oxygen | O | 8 | |
| 3,98 | Fluorine | F | 9 | |
| Helium | He | 2 | ||
| Neon | Ne | 10 | ||
| Argon | Ar | 18 | ||
| Rubidium | Rb | 37 | ||
| Europium | Eu | 63 | ||
| Terbium | Tb | 65 | ||
| Holmium | Ho | 67 | ||
| Hafnium | Hf | 72 | ||
| Radium | Ra | 88 | ||
| Seaborgium | Sg | 106 | ||
| Bohrium | Bh | 107 | ||
| Hassium | Hs | 108 | ||
| Meitnerium | Mt | 109 | ||
| Darmstadtium | Ds | 110 | ||
| Roentgenium | Rg | 111 | ||
| Ununbium | Uub | 112 | ||
| Ununtrium | Uut | 113 | ||
| Ununquadium | Uuq | 114 | ||
| Ununpentium | Uup | 115 | ||
| Ununhexium | Uuh | 116 | ||
| Ununseptium | Uus | 117 | ||
| Ununoctium | Uuo | 118 |
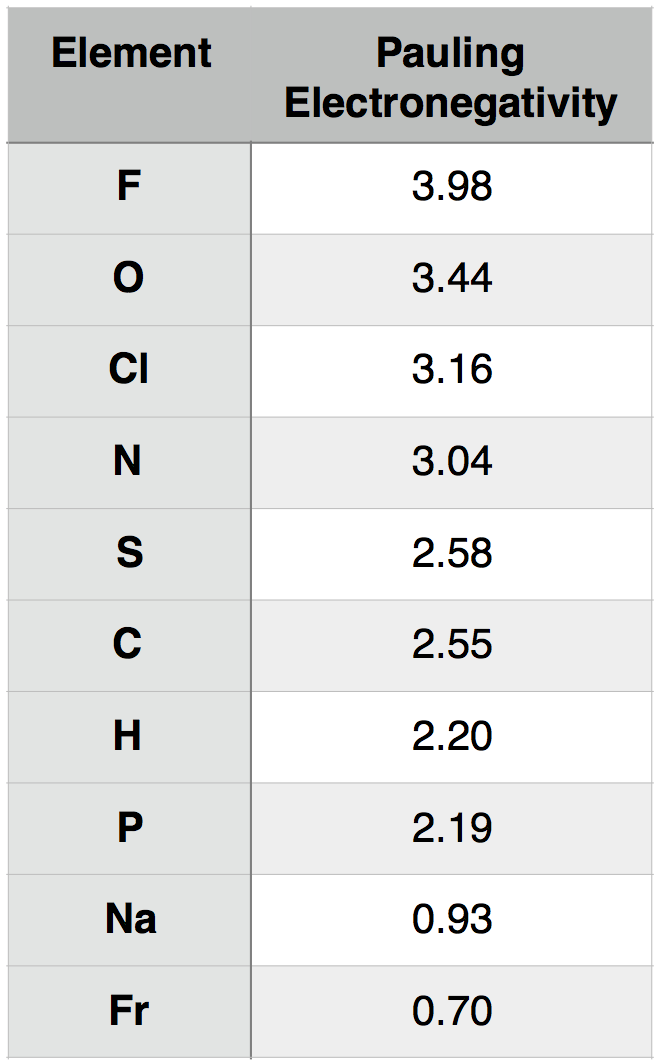
Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. The Pauling scale is the most commonly used. Fluorine (the most electronegative element) is assigned a value of 4.0, and values range down to caesium and francium which are the least electronegative at 0.7. Fluorine is the most electronegative element because the definition of electronegativity makes it so. The electronengativity scales are defined based on experimentally determined properties of the elements. Fluorine has appropriate values for all of the common scales to ensure it has the highest electronegativity. Fluorine is the most electronegative element on the periodic table. Its electronegativity value is 3.98.
We measure electronegativity on several scales. The most commonly used scale was designed by Linus Pauling. According to this scale, fluorine is the most electronegative element with a value of 4.0 and cesium is the least electronegative element with a value of 0.7. Check out the electronegativity values of elements in the electronegativity chart.
Click here: for a schematic overview of the periodic table of elements in chart form
Please report any accidental mistake in the above statistics on chemical elements
Lenntech (European Head Office)
Distributieweg 3
2645 EG Delfgauw
The Netherlands
Phone: +31 152 610 900
fax: +31 152 616 289
e-mail: info@lenntech.com
Lenntech USA LLC (Americas)
5975 Sunset Drive
South Miami, FL 33143
USA
Phone: +1 877 453 8095
e-mail: info@lenntech.com
Table Of Electronegativity
Lenntech DMCC (Middle East)
Level 5 - OFFICE #8-One JLT Tower
Jumeirah Lake Towers
Dubai - U.A.E.
Phone: +971 4 429 5853
e-mail: info@lenntech.com
Copyright © 1998-2021 Lenntech B.V. All rights reserved
Periodic Trends — Electronegativity
| 1A | 2A | 3A | 4A | 5A | 6A | 7A | 8A | |||||||||||
| (1) | (2) | (13) | (14) | (15) | (16) | (17) | (18) | |||||||||||
| 3B | 4B | 5B | 6B | 7B | — | 8B | — | 1B | 2B | |||||||||
| (3) | (4) | (5) | (6) | (7) | (8) | (9) | (10) | (11) | (12) | |||||||||
| 1 | H 2.20 | He n.a. | ||||||||||||||||
| 2 | Li 0.98 | Be 1.57 | B 2.04 | C 2.55 | N 3.04 | O 3.44 | F 3.98 | Ne n.a. | ||||||||||
| 3 | Na 0.93 | Mg 1.31 | Al 1.61 | Si 1.90 | P 2.19 | S 2.58 | Cl 3.16 | Ar n.a. | ||||||||||
| 4 | K 0.82 | Ca 1.00 | Sc 1.36 | Ti 1.54 | V 1.63 | Cr 1.66 | Mn 1.55 | Fe 1.83 | Co 1.88 | Ni 1.91 | Cu 1.90 | Zn 1.65 | Ga 1.81 | Ge 2.01 | As 2.18 | Se 2.55 | Br 2.96 | Kr 3.00 |
| 5 | Rb 0.82 | Sr 0.95 | Y 1.22 | Zr 1.33 | Nb 1.60 | Mo 2.16 | Tc 1.90 | Ru 2.20 | Rh 2.28 | Pd 2.20 | Ag 1.93 | Cd 1.69 | In 1.78 | Sn 1.96 | Sb 2.05 | Te 2.10 | I 2.66 | Xe 2.60 |
| 6 | Cs 0.79 | Ba 0.89 | La 1.10 | Hf 1.30 | Ta 1.50 | W 2.36 | Re 1.90 | Os 2.20 | Ir 2.20 | Pt 2.28 | Au 2.54 | Hg 2.00 | Tl 1.62 | Pb 2.33 | Bi 2.02 | Po 2.00 | At 2.20 | Rn n.a. |
| 7 | Fr 0.70 | Ra 0.89 | Ac 1.10 | Rf n.a. | Db n.a. | Sg n.a. | Bh n.a. | Hs n.a. | Mt n.a. | Ds n.a. | Rg n.a. | Uub n.a. | — | Uuq n.a. | — | — | — | — |
| 6 | Ce 1.12 | Pr 1.13 | Nd 1.14 | Pm 1.13 | Sm 1.17 | Eu 1.20 | Gd 1.20 | Tb 1.10 | Dy 1.22 | Ho 1.23 | Er 1.24 | Tm 1.25 | Yb 1.10 | Lu 1.27 | ||||
| 7 | Th 1.30 | Pa 1.50 | U 1.38 | Np 1.36 | Pu 1.28 | Am 1.30 | Cm 1.30 | Bk 1.30 | Cf 1.30 | Es 1.30 | Fm 1.30 | Md 1.30 | No 1.30 | Lr 1.30 |
Electronegativities reported in Pauling units
Data taken from John Emsley, The Elements, 3rd edition. Oxford: Clarendon Press, 1998.
Electronegativity refers to the ability of an atom to attract shared electrons in a covalent bond. The higher the value of the electronegativity, the more strongly that element attracts the shared electrons.
The concept of electronegativity was introduced by Linus Pauling in 1932; on the Pauling scale, fluorine is assigned an electronegativity of 3.98, and the other elements are scaled relative to that value. Other electronegativity scales include the Mulliken scale, proposed by Robert S. Mulliken in 1934, in which the first ionization energy and electron affinity are averaged together, and the Allred-Rochow scale, which measures the electrostatic attraction between the nucleus of an atom and its valence electrons.

Electronegativity varies in a predictable way across the periodic table. Electronegativity increases from bottom to top in groups, and increases from left to right across periods. Thus, fluorine is the most electronegative element, while francium is one of the least electronegative. (Helium, neon, and argon are not listed in the Pauling electronegativity scale, although in the Allred-Rochow scale, helium has the highest electronegativity.) The trends are not very smooth among the transition metals and the inner transition metals, but are fairly regular for the main group elements, and can be seen in the charts below.
The difference in electronegativity between two bonded elements determines what type of bond they will form. When atoms with an electronegativity difference of greater than two units are joined together, the bond that is formed is an ionic bond, in which the more electronegative element has a negative charge, and the less electronegative element has a positive charge. (As an analogy, you can think of it as a game of tug-of-war in which one team is strong enough to pull the rope away from the other team.) For example, sodium has an electronegativity of 0.93 and chlorine has an electronegativity of 3.16, so when sodium and chlorine form an ionic bond, in which the chlorine takes an electron away from sodium, forming the sodium cation, Na+, and the chloride anion, Cl-. Particular sodium and chloride ions are not 'tied' together, but they attract each other very strong because of the opposite charges, and form a strong crystal lattice.

When atoms with an electronegativity difference of less than two units are joined together, the bond that is formed is a covalent bond, in which the electrons are shared by both atoms. When two of the same atom share electrons in a covalent bond, there is no electronegativity difference between them, and the electrons in the covalent bond are shared equally — that is, there is a symmetrical distribution of electrons between the bonded atoms. These bonds are nonpolar covalent bonds. (As an analogy, you can think of it as a game of tug-of-war between two equally strong teams, in which the rope doesn't move.) For example, when two chlorine atoms are joined by a covalent bond, the electrons spend just as much time close to one chlorine atoms as they do to the other, and the resulting molecule is nonpolar:
When the electronegativity difference is between 0 and 2, the more electronegative element attracts the shared more strongly, but not strongly enough to remove the electrons completely to form an ionic compound. The electrons are shared unequally — that is, there is an unsymmetrical distribution of electrons between the bonded atoms. These bonds are called polar covalent bonds. The more electronegative atom has a partial negative charge, d-, because the electrons spend more time closer to that atom, while the less electronegative atom has a partial positive charge, d+, because the electrons are partly (but not completely) pulled away from that atom. For example, in the hydrogen chloride molecule, chlorine is more electronegative than hydrogen by 0.96 electronegativity units. The shared electrons spend more time close to the chlorine atom, making the chlorine end of the molecule very slightly negative (indicated in the figure below by the blue shaded region), while the hydrogen end of the molecule is very slightly positive (indicated by the red shaded region), and the resulting molecule is polar:
The Most Electronegative Atoms On The Periodic Table
For molecules with more than one covalent bond, the three-dimensional shape of the molecule and how the polar bonds are oriented with respect to each other, determines whether or not the molecule is polar. This polarity of molecules plays a large role in determining the physical properties of compounds.
What Are The Most Electronegative Atoms
